
AFRILCATE
WHAT IS ACID RAIN?
Acid rain is any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms.
Acid rain is also called acid deposition.
Contrary to popular belief, acid rain doesn’t refer to only rains that are acidic, the term covers every acidic deposition that falls from the atmosphere.
This means snow, fog, hail or even dust can be called acid rain if it has a low ph level.
In this form, it’s called dry deposition but in the liquid form, it’s wet deposition.
Inother words, both dry and wet deposition can be called acid rain if it has a low pH value.
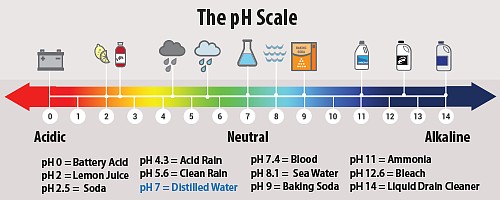
Can you recall the pH scale?
Pure water has a pH of 7, a neutral value.
Anything below that is acidic while above is basic.
Normal rainwater which is considered clean is a little bit acidic with a pH of about 5.2 meaning its slightly acidic.
But, rainwater can also have elevated levels of hydrogen ion with pH within the range of 4.2 -4.5.
In 2000, the most acidic rain that fell in the United States had a pH of 4.3.
WHAT ARE THE CAUSES OF ACID RAIN?
Acid rain is caused by emissions of sulfur dioxide (SO2) and nitrogen oxide (NOx) in the atmosphere.
The SO2 and NOx react with water droplets, oxygen and other chemicals in the atmosphere to form sulfuric and nitric acids.
When the cloud becomes saturated, the precipitate mixture then falls to the ground as acid rain.
| Recall: nitrogen oxide is the combination of NO and NO2 |
Sulfur dioxide and nitrogen oxide are produced from either man-made sources or natural sources.
Man-made sources account majorly for the release of these gases, it includes:
- The burning of fossil fuels by power-production industries releases sulfur into the air (accounts for 2/3 of SO2 and 1/4 of NOx )
- Exhausts from cars cause the formation of nitrogen oxides in the air.
The natural source includes:
- Volcanoes: Sulphur dioxide is produced by volcanic eruptions.
- Lightening: produces nitrogen oxide
- Decaying vegetation
These gases dissolve in water vapor in the cloud to form acid rain.
When gasses like SO2 and NOx are released into the atmosphere, It can be blown by the wind over long distances and across borders.
This makes acid rain a problem for everyone and not just those who live close to the gas emission source.
EFFECTS OF ACID RAIN
There are numerous harmful effects of acid rain most especially on aquatic bodies.
- Acid rain enters water bodies as runoff. This makes water bodies toxic to crayfish and other aquatic animals.
- The lowered pH level of acid rain prevents the hatching of fish eggs and kill parent fishes.
- In an interconnected ecosystem, the rest of the food chain and non-aquatic species like birds are often affected negatively through food poisoning.
- Acid rain harms forests by damaging trees and leaves.
- It causes corrosion of steel structures like bridges.
- Nitrogen oxides present in acid rain contribute to the formation of ground-level ozone, a major pollutant that’s harmful to humans.

WHAT CAN BE DONE?
Since 1990, the Environmental Protection Agency has required companies that emit these two chemicals causing acid rain to make major reductions in their emissions.
Individually, you can also play a role to mitigate acid rain.
Any step you can take to conserve energy will reduce the amounts of fossil fuels that are burned to produce that energy, thereby reducing the formation of acid rain.
This also includes turning off your bulbs, computer, tv set when not in use.
By investing in renewable energies, we can also reduce unnecessary air pollution.

