
AFRILCATE
WHAT IS THERMAL CONDUCTION?
Thermal conduction is the mode of heat transfer by which heat is transferred in a material medium through the microscopic movement of the particles (molecules, atoms, electrons) in the medium without physically transporting the material from one position to another.
This means that thermal conduction doesn’t involve macroscopic movement, i.e bulk fluid movement.
Conduction requires the existence of a material medium before the heat can be transferred, unlike radiation where heat can be transferred through a vacuum and doesn’t need a material medium for its propagation.
Heat is thermally conducted in material from high-temperature areas to low-temperature areas.
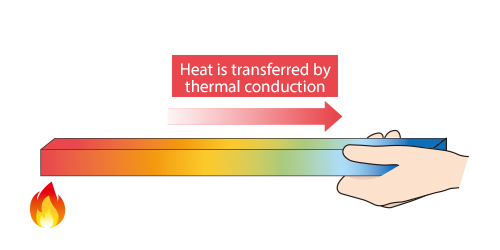
Heat is thermally conducted along the rod.
MICROSCOPIC AND MACROSCOPIC TRANSPORT
Let’s differentiate between these two terms to avoid confusion.
Microscopic transport: This is the movement of micro-particles.
Microparticles are tiny and can’t be seen with the human eyes. They include molecules, atoms and electrons
Macroscopic transport: This is the physical movement of a material from one position to another. It’s also referred to as bulk transport.
It’s important to point out again that conduction involves microscopic transport of particles and not macroscopic transport.
Clear right?
Let’s continue..
KINETIC THEORY OF MATTER AND CONDUCTION
According to the kinetic theory of matter, molecules vibrate about their mean positions in solids.
These molecules travel at different velocities depending on their kinetic energy.
The mean kinetic energy is proportional to the absolute temperature.
Molecules at higher temperatures have higher kinetic energy and collide faster with molecules that have lower kinetic energy.
As a result of this, heat is transferred from higher K.E – molecules to lower K.E molecules.
The increase in kinetic energy of the lower K.E molecules will lead to a corresponding increase in temperature.
Heat will continue to flow across molecules as long as a thermal difference exists.
A point is reached when the thermally conducted heat across all molecules exists at the same temperature.
This is known as thermal equilibrium.
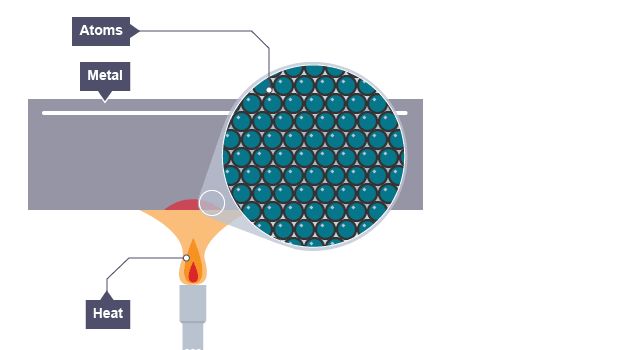
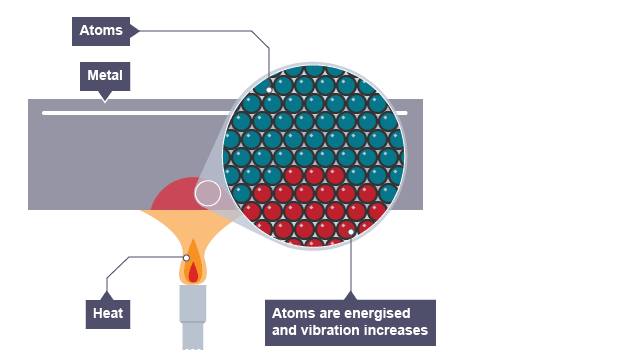
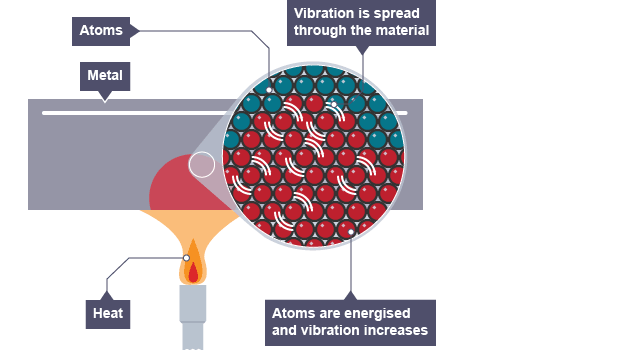
PICTORIAL REPRESENTATION OF THERMAL CONDUCTION
Stage 1
Stage 2
Stage 3
THERMAL CONDUCTIVITY
Thermal conductivity refers to how highly or poorly a medium conducts heat. The symbol for thermal conductivity is k.
Thermal conductivity in metals varies depending on the nature of the material.
There are no free electrons in insulators.
Conduction in insulators is governed only by molecular motion and therefore their thermal conductivities are consequently low.
For liquids and gases, the probabilities of collisions are lower than in solids because they have a longer mean free path.
Therefore, liquids and gases possess lower thermal conductivity when compared with solid.
Also, the molecules in liquids have higher chances of collision compared to gasses. This means that gases have lesser thermal conductivity than liquids.
SOLIDS —> LIQUIDS —> GASES
Decreasing order of thermal conductivity
Thermal conductivity provides an indication of the rate at which heat energy is transferred through a medium by the conduction process.
FOURIER’S LAW OF CONDUCTION
Fourier’s law is a particular law of conduction, formulated by Joseph Fourier in 1922.
It helps us determine the parameters required for heat flow or heat transfer through a conducting medium.
Fourier’s law states that:
“The heat flow rate per unit cross-sectional area A, is proportional to the average temperature gradient and it’s in the direction of decreasing temperature”.
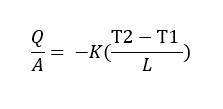
Where k is the constant of proportionality and it’s the Thermal Conductivity of the material.
The heat flow rate per unit area (Q/A) is known as the heat flux.
Obtaining the heat flux involves knowing the individual temperature or temperature difference, the geometry and the thermal conductivity of the object.
If given the heat flux and thermal conductivity of a material, we can determine the temperature difference using Fourier’s law of conduction.
SUMMARY OF THERMAL CONDUCTION
Thermal conduction is the mode of heat transfer through the microscopic movement of particles.
Conduction requires the existence of a material medium before heat can be transferred.
Thermal conduction doesn’t require the physical movement of the material from one location to another (Macroscopic transport).
Thermal conductivity refers to how highly or poorly a medium conducts heat.
It is an indication of the rate at which heat energy is transferred through a medium by conduction process.
Thermal conductivity highest in solids and lowest in gases.
Fourier’s law of conduction states that “The heat flux, q is proportional to the negative average temperature gradient and it’s in the direction of decreasing temperature”.
Fourier’s law is useful for determining the heat flow rate, heat flux, thermal conductivity and temperature difference if given the necessary parameters.

